Effect of Temperature on Oxygen Reduction Reaction Kinetics for Pd Core−Pt Shell Catalyst with Different Core Size
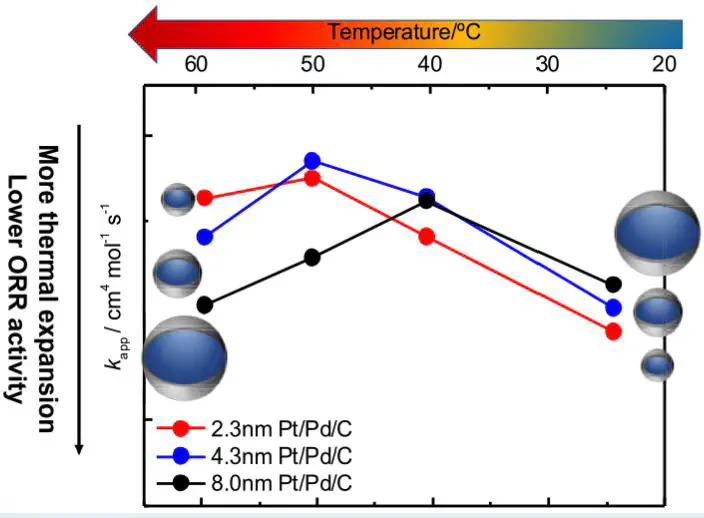 Image credit: Unsplash
Image credit: UnsplashAbstract
The oxygen reduction reaction (ORR) activity and the coverage of oxide species over a Pd core–Pt shell catalyst on carbon support (Pt/Pd/C) with various Pd core sizes (2.3, 4.3, and 8.0 nm) were investigated in the temperature range from 25 to 60 °C and compared with a Pt/C catalyst (TEC10V30E, Tanaka Kikinzoku Kogyo). The apparent rate constant (kapp) of Pt/Pd/C increased with increasing core size at 25 °C. However, kapp of Pt/Pd/C started to decrease at 50–60 °C, while that of Pt/C behaved according to the general Arrhenius equation. Eventually, the 2.3 nm core showed the highest kapp at 60 °C, and the 8.0 nm core was almost the same as that of Pt/C. According to the electrochemical measurements, the coverage of oxide species on Pt/Pd/C was quite smaller than that of Pt/C. However, it increased dramatically with increasing temperature from 25 to 60 °C. Among the Pt/Pd/C, the 8.0 nm core showed the most obvious oxide coverage increase at 60 °C, which was almost identical to that of Pt/C. In contrast, the 2.3 nm core showed the lowest oxide coverage at 60 °C, which was expected to be the cause of the largest kapp. Operando X-ray absorption spectroscopy indicated that the Pt–Pt bond length in Pt/Pd/C was shorter than that in the Pt/C at 25 °C due to compressive surface strain from the Pd core, which is the reason why Pt/Pd/C has higher activity than Pt/C. On the other hand, as Pd has a thermal expansion coefficient higher than that of Pt, Pt/Pd/C showed a Pt–Pt bond length larger than that of Pt/C at 60 °C. A longer Pt–Pt bond length extension was observed at 60 °C in the 8.0 nm core compared to that in the other catalysts.
Add the publication’s full text or supplementary notes here. You can use rich formatting such as including code, math, and images.